Wholesome meals
Most popular this month
“This magical, marvelous food on our plate, this sustenance we absorb, has a story to tell. It has a journey.”
– Joel Salatin
Delicious steamed chicken dish
Our Best Chicken Recipes
Latin Food: A Savory Journey Through Latin American Cuisine
Latin food, also known as Latin American cuisine, is a delectable and diverse culinary journey [...]
8 Best Vegan Latin Food Options: Exploring Delicious Plant-Based Alternatives
Latin gastronomy is, like, super famous for its unique flavors and a huge variety of [...]
The best Shredded Chipotle Beef you can make at home
Welcome to our food blog, where we share delightful recipes that will tantalize your taste [...]
Delicious & Easy Recipe: Mexican Shrimp Bowl with Corn & Black Bean Salsa
Are you in search of a quick and flavorful meal? Look no further! In this [...]
2 Healthy Mango Lime Sorbet Recipes for Your Sweet Tooth
Get ready to dig in a delicious dessert that doesn’t have to compromise your dietary [...]
The best brisket tacos with street corn and jalapeno lime ranch recipe
Tacos are a popular and delicious dish that can be customized in various ways. This [...]
Best Potato Salad Recipe: Asparagus and Mustard Potato Salad
Introducing a delightful dish that’s perfect for the spring and summer seasons – Asparagus and [...]
Best Spicy Potato Wedges Recipe and 5 other types
Spicy Potato Wedges are a popular and delicious side dish that can be served with [...]
Kitchen Tips
Help everything easier
Top 7 Best Blender For Protein Shakes
The best blender for protein shakes is simple to utilize and clean, and it includes [...]
Do Probiotics Make You Poop ? | Best things You Need to Know
This article will answer the question “do probiotics make you poop” as well as explain [...]
Best Way to Store Grapes – To Keep Them “Always” Fresh
Grapes are a fantastic little fruit. You can put them in fruit dishes and salads, [...]
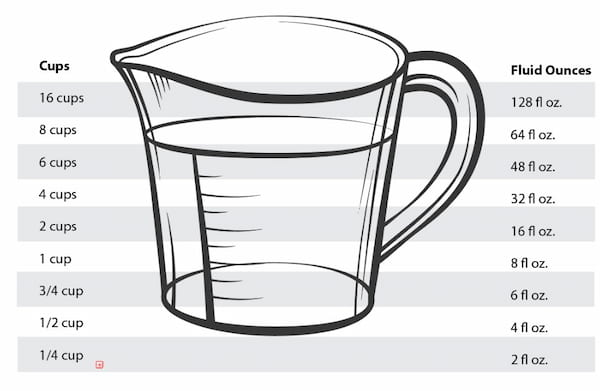
How Many Cups Is 32 Oz
Calculation and measurement are always forms of number conversion that require people to be meticulous [...]